The scientific journal Insectes Sociaux has become “PCI-friendly” in partnership with Peer Community In (https://peercommunityin.org/) — an opportunity to speed up and increase the transparency of peer review, without automatic acceptance. Joël Meunier, who initiated the rapprochement between the journal and PCI, explains what this entails.

IS: Hello Joël, could you introduce yourself ?
Sure! I’m a CNRS researcher at the University of Tours, France, where I explore how social life evolves in insects. After completing a PhD on ants in Lausanne, I shifted my focus more than 15 years ago to earwigs — which, despite their modest reputation, have surprisingly rich and fascinating family and social behaviors. I’ve been serving as an associate editor for Insectes Sociaux for the past few years, and I’ve been involved with the Peer Community In (PCI) initiative since its launch in 2017.

IS: In simple terms, what is the PCI (Peer Community In) initiative and how does it work, from uploading a preprint to receiving a recommendation?
PCI is a free, scientist-run initiative that offers open and transparent peer review for preprints: basically, research papers shared publicly before formal journal publication. It was created to tackle some big issues in academic publishing: skyrocketing publication fees, the declining value of peer review for authors, and the overemphasis on journal prestige rather than the science itself. The idea is simple: to offer high-quality, community-driven peer review at no cost, across fields such as zoology, ecology, evolutionary biology, paleontology, and many others.
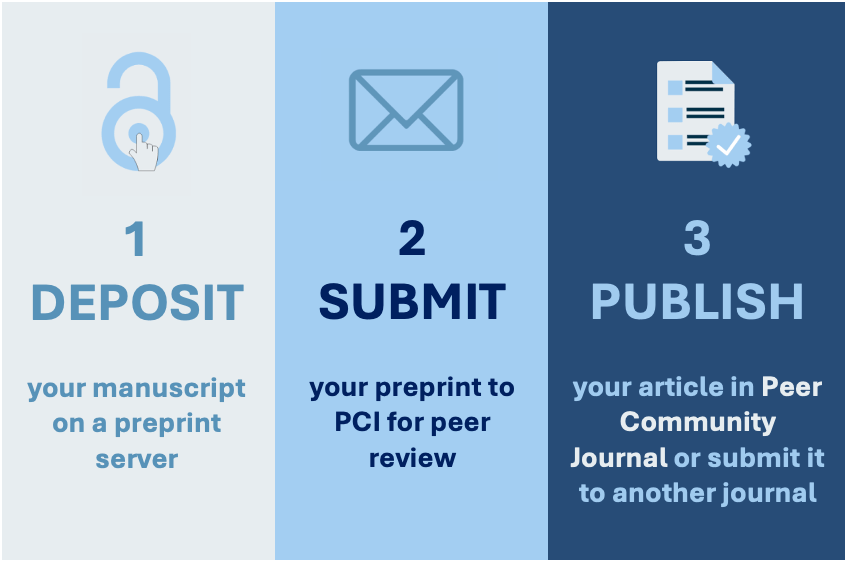
How does it work? It’s pretty straightforward and fully detailed on the PCI website (https://peercommunityin.org/submit-a-preprint-to-pci/), but in brief:
- You upload your manuscript to a preprint server (like bioRxiv) and your data to a public repository.
- You submit the link to the relevant PCI community (e.g., PCI Zoology).
- The PCI team checks your submission for FAIR data compliance, then sends it to “recommenders” (who act like editors). They organize peer review, and if your manuscript meets PCI’s standards (usually after revisions) the recommender writes a short summary explaining why it’s worth recommending.
- Your preprint, along with all reviews and responses, gets an official DOI and stays openly accessible. You can then submit your recommended preprint to journals, including those like Insectes Sociaux that recognize PCI’s process.
In short, PCI helps researchers get constructive feedback and recognition for their work, all while keeping science open and accessible.
IS: How does PCI differ from the traditional peer-review process? What are its main strengths?
PCI follows a fairly traditional peer-review process: each manuscript is reviewed by at least two reviewers, and several rounds of revision may be needed before a recommendation is granted.
That said, there are some important differences.
First, PCI delivers a recommendation, not a publication. In other words, your manuscript receives a kind of quality stamp (complete with a DOI) but it is not tied to a specific journal. After that, you can either submit the improved manuscript (together with the previous reviews) to a PCI-friendly journal, which can take those reviews into account to avoid unnecessarily additional rounds of reviews, or submit it to the PCI Journal.
Second, recommenders must write a short text explaining why the manuscript is being recommended. This encourages real engagement: It’s not just clicking “accept.” It also means that potential recommenders can decline early on if they feel they won’t be able to fully commit to the process. In that sense, it adds responsibility and transparency.
Finally (and this is my personal experience) the reviews are often of very high quality. Because the manuscript is not competing for space in a specific journal, reviewers focus on the science itself rather than on whether it feels “flashy” enough. The feedback tends to be constructive, detailed, and genuinely helpful.
IS: Does the “PCI-friendly” status mean that authors must now deposit a preprint and go through PCI in order to submit to Insectes Sociaux?
No, absolutely not. Authors are still welcome to submit directly to Insectes Sociaux through the regular process. Being PCI-friendly simply means that authors have the option of going through PCI first, and that doing so will not delay publication if they later submit to the journal. It’s an opportunity — not an obligation — to support open science at no extra cost (in terms of fees, time, or energy) and potentially improve the manuscript and the reproducibility of its results along the way.
IS: What specific documents should an author provide when submitting via PCI (recommendation, review reports, author responses), and how does that make submission to the journal easier?
Once your manuscript has been recommended by PCI, you simply follow the usual submission procedure for Insectes Sociaux. In your cover letter, you mention that the manuscript has been recommended by PCI and include the DOI of the PCI recommendation. That’s it. Everything is already accessible through the DOI, which makes the process straightforward and transparent.
IS: What concrete benefits does PCI offer authors (visibility, speed, recognition for reviewers) and the Insectes Sociaux community?
For authors, the benefits are multiple. First, they receive a transparent, high-quality peer-review process that helps improve the manuscript — which they can still submit to the journal of their choice. In some cases, the reviewers’ enthusiasm may even encourage authors to aim for a broader audience than they initially considered. Second, a PCI recommendation gives early and broad visibility to the work, even before journal publication. Finally, it places the community working on social insects at the forefront of open science — and that’s something we can be proud of.
IS: What concrete impact do you expect for the journal’s readers and authors (e.g. decision times, quality of submissions)?
I expect a very positive impact overall. First, manuscripts that come through PCI will often have already gone through a thorough and constructive review process, which should translate into stronger, clearer, and more robust papers. That ultimately benefits everyone: readers get higher-quality science, and authors submit work that has already been improved through detailed feedback. Importantly, this shouldn’t lead to longer decision times. On the contrary, because the reviews are already available and transparent, the evaluation process at the journal can be more efficient. And all of this comes without additional publication costs for authors. In that sense, it’s really a win-win situation: better papers, no extra fees, and no unnecessary delays.
IS: To conclude: what practical advice would you give a young researcher who is unsure whether to post a preprint + use PCI or to submit directly to Insectes Sociaux?
Academic publishing is clearly evolving, and open science is playing an increasingly important role in our careers. For a young researcher who is hesitating, my advice would be simple: give it a try. Posting a preprint and going through PCI can be a very positive experience. You receive constructive feedback in a transparent setting, your work becomes visible earlier, and you engage directly with the scientific community. It’s not a risky move; it’s an opportunity to strengthen your manuscript and to take part in a more open and collaborative way of doing science. At the end of the day, it’s about finding the process that suits you best, but PCI is definitely worth experiencing at least once.
IS: Thank you Joël! Oh and… to all our readers, please have a look to the Joël research here!